Company Overview
 While enrolled in the Harvard-MIT Health Sciences and Technology program, Vadim Backman started to “think big.” He expanded his childhood goal of becoming a physician to help people; now he dreamed of saving millions of lives through the development of advanced technology. Dr. Backman believed that a team of experts that shared his vision could have as significant an impact on several cancer types as the Pap smear has had in cervical cancer. To realize his dream, he created the Backman Photonics Laboratory at Northwestern University.
While enrolled in the Harvard-MIT Health Sciences and Technology program, Vadim Backman started to “think big.” He expanded his childhood goal of becoming a physician to help people; now he dreamed of saving millions of lives through the development of advanced technology. Dr. Backman believed that a team of experts that shared his vision could have as significant an impact on several cancer types as the Pap smear has had in cervical cancer. To realize his dream, he created the Backman Photonics Laboratory at Northwestern University.
Recognizing their shared values and their ability to collaborate effectively, in 2011 Drs. Backman, Roy and Subramanian created NanoCytomics, a team of professionals dedicated to enabling dramatic improvements in cancer survival rates.
The proprietary PWS technology platform is designed to provide highly accurate, low-cost, non-invasive testing that will revolutionize the risk stratification of patients, helping physicians identify people that are likely to benefit from gold-standard procedures to diagnose cancer as early as possible, when treatments are most likely to be effective.
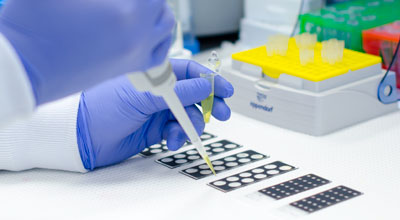
 Of particular significance will be the simple, easy-to-implement sample collection process that will be able to be performed in the primary care physician’s office during a routine physical examination. Samples will be sent from the physician’s office to a testing laboratory for analysis with NanoCytomic’ PWS technology platform. Within a few days of sample collection, NanoCytomics will send test results to the ordering physician, who can use the results to determine whether or not the patient is likely to benefit from gold-standard diagnostic tests, such as colonoscopies, CT scans, and biopsies.
Of particular significance will be the simple, easy-to-implement sample collection process that will be able to be performed in the primary care physician’s office during a routine physical examination. Samples will be sent from the physician’s office to a testing laboratory for analysis with NanoCytomic’ PWS technology platform. Within a few days of sample collection, NanoCytomics will send test results to the ordering physician, who can use the results to determine whether or not the patient is likely to benefit from gold-standard diagnostic tests, such as colonoscopies, CT scans, and biopsies.
The benefits of this risk-stratification testing platform are twofold:
These are “think-big goals” that the NanoCytomics team believes it is well positioned to accomplish over the coming years. The company anticipates offering its first commercially available Laboratory Developed Test (LDT) for cancer risk stratification in 2018.







